Chemistry 2023
Objective Questions
Which of the following laws or theory cannot be explained by the application of the kinetic theory of gases?
Correct Answer: A) Dalton's atomic theory
The primary component of natural gas is
Correct Answer: C) Methane
Thermal cracking of alkanes usually
Correct Answer: A) Involves decomposition
Carbon is deposited in the exhaust pipes of cars because of
Correct Answer: D) Incomplete combustion of petrol
How many moles of copper would be deposited by passing 1 Faraday of electricity through a CuCl₂ solution?
Correct Answer: C) 0.5
Alkenes can be manufactured by
Correct Answer: B) The cracking of hydrocarbons
Petrochemistry is an example of
Correct Answer: B) Applied chemistry
Species that occur in a reaction pathway but not in the overall reaction are known as
Correct Answer: D) Intermediates
Which of the following statements is correct?
Correct Answer: A) An alkane with 49 carbon atoms contain 100 hydrogen atoms
The best indicator to use for the titration of ethanoic acid with sodium hydroxide is
Correct Answer: C) Phenolphthalein
The reduction half equation of the
following reaction is:
Zn(s) + CuSO₄(aq) → ZnSO₄(aq)
+ Cu(s)
Correct Answer: B) CuSO₄(s) + H₂O(l) → Cu²⁺(aq) + SO₄²⁻(aq)
If 100 cm³ of a saturated solution of sodium tetraoxosulphate (VI) at 30°C contains 10.5 g of the salt, what would be its solubility at this temperature? [Na₂SO₄ = 142]
Correct Answer: A) 0.74 mol dm⁻³
An example of a crystalline substance that does not possess water of crystallization is
Correct Answer: A) Potassium trioxonitrate
The salt solution formed from a reaction between ethanoic acid and sodium hydroxide solution would be
Correct Answer: A) Basic
Which of the following reactions represents the hydrolysis of an alkanoate?
Correct Answer: C) CH₃COOC₂H₅ + H₂O ⇌ CH₃COO⁻ + CH₃CH₂OH
Which of the following statements about the collision theory is correct?
Correct Answer: B) Rate of reaction is proportional to the number of effective collisions
Why are H₂SO₄ and CaCl₂ not suitable for drying ammonia gas?
Correct Answer: C) React with the gas
Which of the following equations does not illustrate correctly one of the reactions of chlorine?
Correct Answer: C) Cl₂ + 2NaOH → NaCl + NaClO + H₂O
How many unpaired electrons are present in ₂₆Fe³⁺?
Correct Answer: D) 5
Going down Group II in the periodic table normally
Correct Answer: B) Melting point increases
Which of the following elements has its valence electrons in the S-orbital?
Correct Answer: A) Sodium
The periodic property that is used to determine whether a covalent molecule is polar or not is
Correct Answer: C) Electronegativity
The following steps are scientific methods except
Correct Answer: B) Open-mindedness
Isoelectronic species have the same number of
Correct Answer: A) Electrons
An element X has two isotopes, ⁶⁵X₃₀ and ⁶⁶X₃₀ with relative abundance of 60% and 40% respectively. The relative atomic mass of X is
Correct Answer: D) 65.40
The pair of compounds that belongs to the same homologous series is
Correct Answer: D) C₄H₁₀ and C₅H₁₀
A consequence of global warming is
Correct Answer: A) Flooding
Which of the following gases has the lowest rate of diffusion? [H = 1.0, C = 12.0, N = 14.0, O = 16.0]
Correct Answer: D) Oxygen
The gas that is less dense than air is
Correct Answer: B) Nitrogen
Which of the following equimolar solutions has the highest conductivity?
Correct Answer: C) H₂SO₄(aq)
What takes place at the cathode during electrolysis?
Correct Answer: C) Cations are discharged
How many grammes of NaOH (s) would be needed to produce 100.0 cm³ of a 0.20 mol dm⁻³ NaOH(aq)? (NaOH = 40.0)
Correct Answer: B) 0.80 g
The formation of a bond between hydrogen and a highly electronegative atom results in
Correct Answer: A) Polarity
The molecule that has a non-polar covalent bond is
Correct Answer: D) Cl₂
Which of the elements in the table below would react more readily with chlorine?
|
Element |
Ionization energy (KJ mol-1 x 10-3 |
| W | 12.0 |
| X | 21.0 |
| Y | 106.0 |
| Z | 200.0 |
Correct Answer: A) W and X only
The relative molar mass of a gaseous hydrocarbon is 30. Determine its vapour density.
Correct Answer: A) 15
Consider the following reaction
equation:
2SO₃(g) → 2SO₂(g) + O₂(g), ΔH = +198
kJ/mol
Which of the following statements about the
reaction is correct?
Correct Answer: D) 198 kJ of energy is absorbed
Which of the following properties does not give evidence of the kinetic theory of matter?
Correct Answer: C) Polymerization
A compound that could be dried by using conc. tetraoxosulphate (VI) acid and not by calcium oxide is likely to be
Correct Answer: D) An acid anhydride
Positive ions in a sea of electrons are found in
Correct Answer: D) Metallic bonds
Dilute trioxonitrate (V) acid does not produce hydrogen when it reacts with metals because
Correct Answer: A) It is a strong oxidizing agent
How many moles are there in 3.0 g of O₂? [O = 16.0]
Correct Answer: B) 0.0930 moles
Copper (II) ions are able to participate in co-ordinate covalent bonding because they
Correct Answer: C) Have vacant d-orbital
Determine the volume of 0.100 mol of HCl in 0.250 mol/dm³ of solution.
Correct Answer: B) 100 cm³
Which of the statements about gases is not correct?
Correct Answer: D) Gases are highly soluble in water at high temperatures
Arrange the following compounds in decreasing order of their boiling points:
Correct Answer: C) HF, NH₃, SiH₄, CH₄
The following statements are correct except
Correct Answer: D) There is large decrease in the volume of a solid metal when pressure is applied to it
The vapour density of an organic compound with the molecular formula C₂H₄O₂ is [H=1.0, C=12.0, O=16.0]
Correct Answer: D) 30
A mixture containing two salts of different solubility can be separated by
Correct Answer: C) Crystallization
The separation technique that is suitable for separating iodine from tetrachloromethane is
Correct Answer: A) Solvent extraction
Theory Questions
a. What is a transition element?
b. Consider the electron configuration of the following
elements: A=2, 8,6; B=2,8,2; C=2,8,1, D=2,8,8
State the element which forms a: i. doubly charged cation;
ii. soluble trioxocarbonate (IV).
c. Explain briefly why there is a general increase in the
first ionization energies of the elements across the period
in the periodic table.
d. Give two examples of an aliphatic compound
e. Explain briefly why alkanols are stronger bases than
water.
f. State the major raw materials used in the Solvay
process
g. What is geometric isomerism?
h. Give a reason why water gas is a better fuel than
producer gas.
i. Define the term heat of combustion.
ji. State Faraday's second law of electrolysis
ii. Calculate the amount of silver deposited when 10920
coulombs of electricity is passed through a solution of a
silver salt.
[IF = 96500 C mol-1]
a. In an experiment, 20.0 cm3
of a solution containing 4g/dm3
of sodium hydroxide was neutralized by 8.0 cm3
of dilute tetraoxosulphate (VI) acid:
i. Write a balanced equation for the reaction;
ii. calculate the concentration of the acid in mol/ dm3
b
i. State two postulates of the Kinetic theory of gases which
real gases do not obey
ii. Explain briefly why real gases do not obey the
postulates stated in 2(b)(i).
c. Consider the following compound:
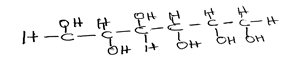
ii. name the two structural isomers of the compound;
iii. state the chemical process involved in the preparation
of the compound from starch,
iv. write the chemical equation for the steps involved in
the process in 2(c)(ii).
v. name two enzymes involved in the process in 2(c)(iii)
d. Explain briefly the term structural isomerism.
a. A compound contains 52.2% C, 13.1 % H and Oxygen only. The
vapour density of the compound is 23.
Determine
i.) its empirical formula;
ii.) its molecular
formula, [H=1.0, C=12.0, O= 16.0]
iii). The compound reacts with sodium metal to produce
hydrogen gas and when warmed with acidified KMnO4(aq)
gives a solution that turns from purple to colorless. It
also forms a sweet-smelling liquid when heated with ethanoic
acid in the presence of concentrated H2SO4
name the functional group present in the compound;
iv). draw the structural formula of the compound.
b. Outline the chemical equations for the production of
ethanol from cooked cassava.
c
i. Explain briefly why a piece of aluminum does not react
with water.
ii. How can a pure sample of aluminum chloride crystals be
prepared from aluminium
d. Describe how water can be separated from aqueous
CuSO4
a. Starting with calcium chloride, describe briefly how a
solid sample of calcium trioxocarbonate (IV) can be prepared
in the laboratory.
b. With relevant equations outline the procedure for the
purification of impure copper.
c. Copper reacts with concentrated trioxonitrate (V) acid:
i. write a balanced chemical equation for the reaction;
ii. state what would be observed in the reaction,
iii. state why the copper is oxidized;
iv. an excess of copper is added to 25.0 cm3 of 16.0 mol dm3
HNO3. Calculate the volume of the gas formed at s.t.p.
[H=1.0, N=14.0, O= 16.0, Cu=63.0; Molar volume of gas at
.s.t.p.=22.4 dm3]
di. Pure HNO3, is a colourless liquid but when exposed to
air, it turns yellowish-brown in colour. Explain briefly
this observation.
ii. Write a balanced equation for the laboratory preparation
of hydrogen trioxonitrate (V) acid
a. Describe how iron and aluminum reacts with each of the
following substances:
i. dilute H2SO4
ii. dilute HNO3
b
i. Write an equation for the burning of sulphur in air.
ii. Name the catalyst used in the contact process.
iii. In the contact process, why is an excess of air
used?
iv. Why is it necessary to cool the catalyst used in
5(b)(ii)?
v. Give a reason why the air used in the contact process
needs to be as clean as possible.
vi. State two reasons why SO2 should not be discharged into
the atmosphere.
c
i. State the reagents and conditions used in the laboratory
preparation of chlorine.
ii. State two uses of chlorine.
d
i. Name the drying agents for each of the following gases:
(I)Hydrogen;
(II)sulphur (IV) oxide;
(III)Ammonia.
ii. State the components of the following
(I)Bronze;
(II). Brass.